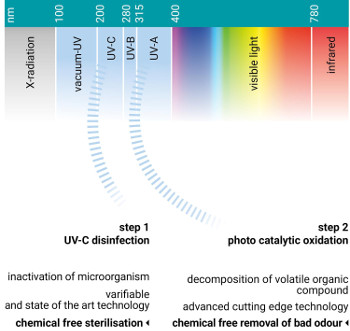
OPERATING PRINCIPLE UVC
Why UV disinfection?
Microorganisms such as bacteria, moulds, yeasts and protozoa can be destroyed or removed by physical, biological and chemical methods. UVC works using a photolytic effect whereby the radiation destroys or inactivates the microorganism so that it can no longer multiply.
For DNA it does this by causing adjacent thymine bases to form a chemical bond thus creating a dimer and if sufficient of these are created DNA cannot replicate. Some microorganisms can repair themselves by absorbing UVA. In other cases UVC (and indeed UVA or UVB) can cause bond splitting in a molecule resulting in the creation of free radicals, which are often highly labile and which can react together to produce an inert end product. For disinfecting these effects are produced by wavelengths below 320 nm, with the optimum effect occurring at around 260 nm. The phenomenon whereby microorganisms can be disfigured or destroyed is independent of host state (fluid or solid) and indeed pH or temperature, the important feature of the action is that radiation can reach the organism; this means that a bacterium shadowed by another or by a particle will escape attack. Unlike other techniques, UVC photolysis rarely produces potentially dangerous byproducts.
Whilst UVC can be used as the exclusive solution in some applications, it is often used in tandem with other techniques. It follows that a single technology solution approach is unlikely to be ideal. It also follows that since UVC is so simple and energy effective, it is perhaps wise to consider this option first.Ultraviolet Radiation
UV radiation belongs to the category of electro-magnetic radiations. Other well known examples of this category are X-ray, light, radio and television radiation. The only difference between the mentioned kinds of electro-magnetic radiations is the special length of the radio waves. The shortest wavelengths belong to the cosmic and roentgen radiation. Very long wavelengths are connected with alternating current. Optic radiation is only a small sector. One of them is UV radiation. Visible und infrared radiations are examples for long wavelengths in this spectrum of waves.
Ultraviolet is that part of electro-magnetic radiation bounded by the lower wavelength extreme of the visible spectrum and the upper end of the X-ray radiation band. The spectral range of ultraviolet radiation is, by definition between 100 and 400 nm (1nm=10-9m) and is invisible to human eyes.Using the CIE classification the UV spectrum is subdivided into three bands:
- UVA (long wave radiation) from 315 nm to 400 nm
- UVB (medium wave radiation) from 280 nm to 315 nm
- UVC (short wave radiation) from 100 nm to 280 nm
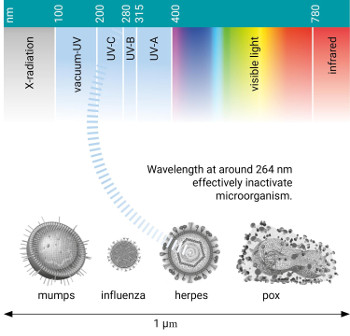
A strong germicidal effect is provided by the radiation in the short wave UVC band. The UV radiation emitted by a source is expressed in watts (W) and the irradiation density is expressed in watts per square metre (W/m²). For germicidal action dose is important. The dose is the irradiation density multiplied by the time (t) in seconds and expressed in joules per square metre (J/m²). 1 joule is 1 watt-second.
Microorganisms effective resistance to UV radiation varies considerably. Moreover, the environment of the particular microorganism greatly influences the radiation dose needed for its destruction. Water, for instance, may absorb a part of the effective radiation depending on the concentration of contaminants in it. Iron salts in solution are well known inhibitors. Iron ions absorb the UV radiation.Why UV disinfection?
Today the most used method of disinfection is chlorination. One problem of this method is the release of halo forms for example chloroform. These substances are suspected to cause cancer. In consequence it is tried to substitute the use of chlorine. In addition the use of water sources near the surface of earth is growing. This water is more germs infected then groundwater. UVC radiation isan effective method to disinfect water without any toxic impact. The principle follows nature. Sun radiation with typical oxidize and germicidal impact is an important factor for cleaning our environment. Doses and effectiveness of artificial UVC radiation is increased significantly in comparison to sun radiation.
OPERATING PRINCIPLE Photo Catalytic Oxidation (PCO)
Why Photo Catalytic Oxidation?
In many applications besides the effective UVC disinfection the removal of bad odour is a real challenge for the process technology. Especially Volatile Organic Compound (VOC) generated by bacteria’s can lead to bad odour (e.g. carbon compound like methane).
Since the pure UV-C disinfection does not change the chemical composition of the treated medium the direct removal of bad odour is not possible.
Therefore for reduction of bad odour the principle of Photo Catalytic Oxidation can be applied in addition to the UV-C disinfection:- Special UVA light activates a Titanium Oxide photo catalyst.
- This process creates hydroxyl radicals and super-oxide ions, which are highly reactive electrons.
- These highly reactive electrons aggressively combine with Volatile Organic Compounds (VOC) – bad odour.
- This breaks the pollutant down into harmless carbon dioxide and water molecules, making the air even more purified.
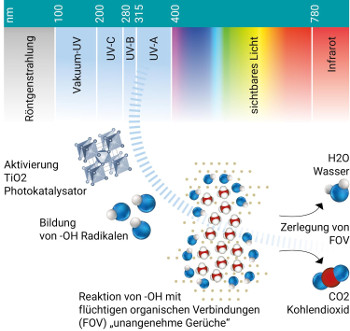
In consequence a considerable reduction of bad odour can be reached via this complementary technology.
It is important to note that the technology of Photo Catalytic Oxidation does not imply the same disinfecting effect like UV-C radiation and cannot replace the technology of UV-C disinfection.
Therefore only the combination of UVC- disinfection and Photo Catalytic Oxidation provides a successful approach to simultaneously reach disinfection and removal of bad odor.
It is therefore part of the PURION philosophy to apply Photo Catalytic Oxidation only in combination with the conventional UV-C disinfection.